A New Maintenance Strategy After GLP Injections
Oral GLP-1 maintenance after injectable weight loss
Eli Lilly has reported positive topline Phase 3 results from ATTAIN-MAINTAIN, a trial designed to answer a very practical question: after achieving weight loss on injectable therapies, can patients maintain that loss by switching to a once-daily oral GLP-1?
Trial Design and Methodology
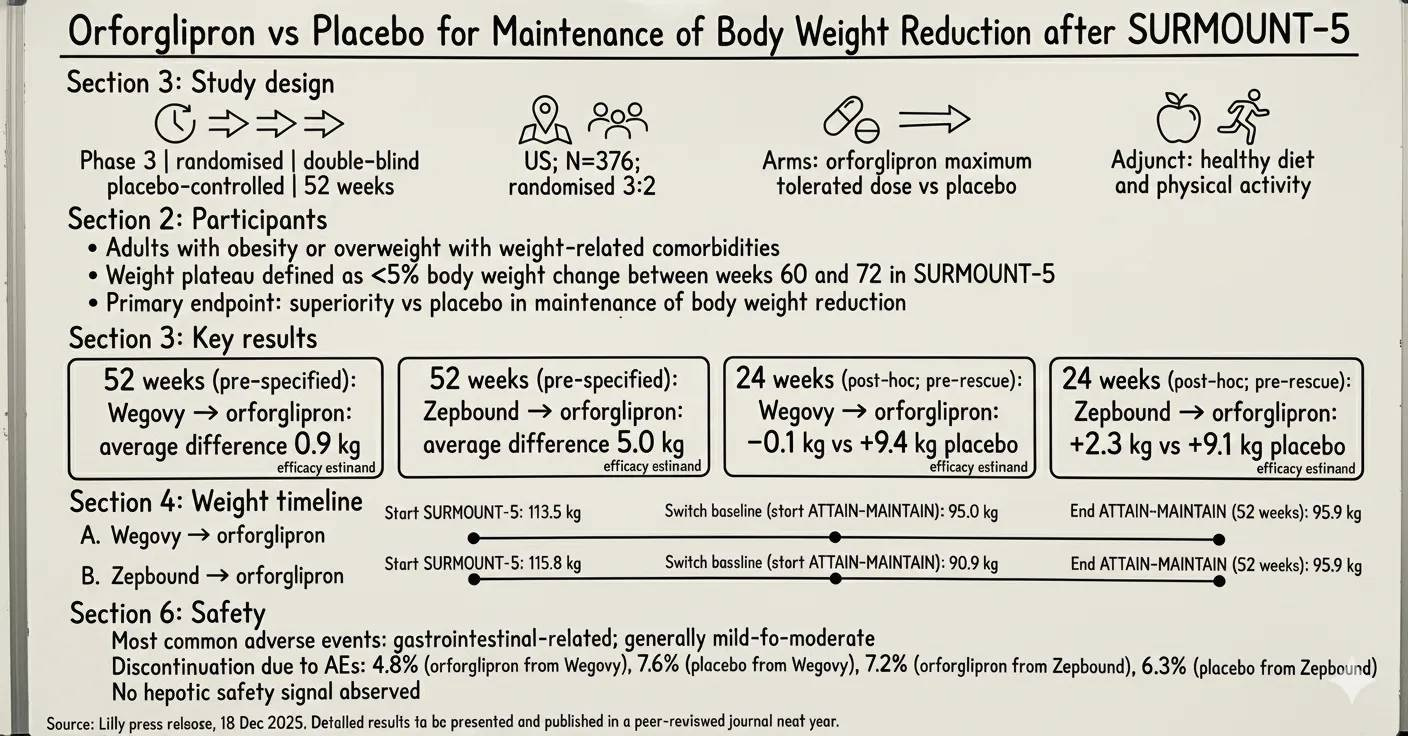
The 52 week trial involved 376 adults with obesity or overweight and related comorbidities. All participants had previously completed the 72 week SURMOUNT-5 trial, where they reached a weight plateau (defined as <5% change between weeks 60 and 72) using the highest tolerated doses of injectable Wegovy or Zepbound
Participants were re-randomised to receive either orforglipron (oral GLP-1) or a placebo as an adjunct to lifestyle interventions
Primary and Secondary Outcomes
Orforglipron met its primary endpoint, demonstrating superior maintenance of body weight reduction compared to placebo at 52 weeks. Key data includes:
Participants who transitioned from Wegovy to orforglipron maintained their weight within an average difference of 0.9 kg.
Participants transitioning from Zepbound to orforglipron maintained their weight within an average difference of 5.0 kg.
In post hoc analyses at 24 weeks, those switching from Wegovy to orforglipron saw a weight change of -0.1 kg, compared to a 9.4 kg gain in the placebo group. Similarly, those switching from Zepbound saw a 2.6 kg change versus a 9.1 kg gain for placebo.
Safety and Tolerability
The safety profile was consistent with previous Phase 3 studies of orforglipron. The most frequently reported adverse events were gastrointestinal-related, typically mild to moderate in severity. Discontinuation rates due to adverse events ranged from 4.8% to 7.2% for those on orforglipron, depending on their previous injectable therapy.
Clinical Implications and Patient Access
As a once daily oral alternative, orforglipron addresses barriers for needle averse patients. This small molecule formulation allows for flexible dosing without food or water restrictions, and it’s scalability could support improved global affordability and treatment access. Having been submitted to the U.S. Food and Drug Administration for regulatory approval under an expedited review process, orforglipron is being evaluated as a practical oral option for chronic weight management and long term maintenance
Ready to Go Beyond the Basics?
The GLP-1 Handbook is your go to reference, filled with clear guidance and patient ready resources designed for immediate application in your consults.
Navigating Weight Loss Medications: A Short Course for Dietitians
Want to build a deeper, strategic understanding? This structured course offers theory and real world learning to solidify your expertise and help you navigate GLP-1s with confidence.
References
Eli Lilly and Company (2025) Lilly’s orforglipron helped people maintain weight loss after switching from injectable incretins Available at: https://investor.lilly.com/news-releases/news-release-details/lillys-orforglipron-helped-people-maintain-weight-loss-after (Accessed: 15 December 2025).